Introduction
Suicide is a growing public health concern with ramifications for families, friends, coworkers, communities and society. The World Health Organization reports that globally, more than 700,000 people die due to suicide every year (World Health Organization, 2023). Depression, anxiety, post-traumatic stress disorder (PTSD) are well-established risk factors for suicide and the emergence of suicide ideation (SI) (Heinrich et al., Reference Heinrich, Hofmann, Baurecht, Kreuzer, Knüttel, Leitzmann and Seliger2022; Turecki et al., Reference Turecki, Brent, Gunnell, O’Connor, Oquendo, Pirkis and Stanley2019; Urban et al., Reference Urban, Rao, Bressel, Neiger, Solomon and Mileshkin2013). The pathophysiology of depression and SI is complex, involving dysregulation of neurotrophic factors like brain-derived neurotrophic factor (BDNF), neuroinflammation and oxidative stress (Guan et al., Reference Guan, Xu, Ji, Wang, Liu, Tang, Gu, Chen, Huang, Liu and Jiang2021). Despite the availability of various treatment options, including medications and psychotherapies, SI remains difficult to treat in some patients, particularly those who are at high risk for suicide (Zalsman et al., Reference Zalsman, Hawton, Wasserman, van Heeringen, Arensman, Sarchiapone, Carli, Höschl, Barzilay, Balazs, Purebl, Kahn, Sáiz, Lipsicas, Bobes, Cozman, Hegerl and Zohar2016).
Ketamine, a noncompetitive N-methyl-D-aspartate receptor antagonist, has been utilized as an anaesthetic since the 1960s (Zhang et al., Reference Zhang, Zhou, Jiang, Li, Ju, Cheng, Zhou and Zhou2023). Besides its anaesthetic properties, ketamine has also been proven to have antidepressant effects (Bartoli et al., Reference Bartoli, Riboldi, Crocamo, Di Brita, Clerici and Carrà2017; Marcantoni et al., Reference Marcantoni, Akoumba, Wassef, Mayrand, Lai, Richard-Devantoy and Beauchamp2020; Yin et al., Reference Yin, Yan, Wei, Sun, Ding, Zhang and Li2024). The antidepressant effects of ketamine are believed to be due to its ability to enhance the production of brain-derived neurotrophic factor, an essential protein for neuronal growth and survival. This aligns with a broader understanding that the hippocampal CRTC1-CREB-BDNF pathway is critical for antidepressant efficacy (Liu et al., Reference Liu, Tang, Ji, Gu, Chen, Huang, Zhao, Sun, Wang, Guan, Liu and Jiang2020). Ketamine has been used off-label for treating depression, anxiety, PTSD and other psychiatric disorders, and has shown quick and prolonged antidepressant effects in some patients (Singh et al., Reference Singh, Fedgchin, Daly, De Boer, Cooper, Lim, Pinter, Murrough, Sanacora, Shelton, Kurian, Winokur, Fava, Manji, Drevets and Van Nueten2016).
The rapid and sustained antidepressant effects of ketamine render it a promising candidate for treating SI. Studies have established that ketamine can promptly diminish SI in patients suffering from depression, bipolar disorder, PTSD and other psychiatric disorders. Moreover, ketamine’s anti-suicidal effects seem to be unrelated to its antidepressant effects, indicating that ketamine may employ distinct mechanisms of action to mitigate SI (Ballard et al., Reference Ballard, Ionescu, Vande Voort, Niciu, Richards, Luckenbaugh, Brutsché, Ameli, Furey and Zarate2014; Grunebaum et al., Reference Grunebaum, Ellis, Keilp, Moitra, Cooper, Marver, Burke, Milak, Sublette, Oquendo and Mann2017).
Despite promising results from individual studies, a consensus on the effectiveness and safety of ketamine in reducing SI has not been reached. Therefore, this study aims to conduct a systematic review and meta-analysis to comprehensively evaluate the existing literature on ketamine treatment for SI in high-risk suicide groups, and to determine the overall efficacy and safety of ketamine in reducing SI in this population. The study will also explore potential moderators of the effect of ketamine on SI, such as dose, frequency of administration, duration of treatment and baseline clinical characteristics. The findings of this review will provide valuable insights into the potential role of ketamine in managing suicide risk, and will inform clinical practice and future research in this area.
Methods
This study complies with PRISMA 2020 recommendations (Page et al., Reference Page, McKenzie, Bossuyt, Boutron, Hoffmann, Mulrow, Shamseer, Tetzlaff, Akl, Brennan, Chou, Glanville, Grimshaw, Hróbjartsson, Lalu, Li, Loder, Mayo-Wilson, McDonald, McGuinness, Stewart, Thomas, Tricco, Welch, Whiting and Moher2021). The protocol was registered in the International Prospective Register of Systematic Reviews database (ID: CRD42023415655).
Eligibility criteria
We included studies if they met the following criteria:
(1). Type of studies: Experimental and observational studies, including randomized trials, open-label, cohort and case-control studies were considered for inclusion. The search was limited to studies published in English.
(2). Type of participants: Individuals identified as high-risk for SI, as determined by various suicide assessment scales, without restrictions on sex, age, or race.
(3). Type of interventions: Treatment with ketamine intervention, which can be performed using any route of administration. For example, intravenous, intramuscular, oral, or inhalation administration.
(4). Type of control: The comparison intervention was routine treatment, no treatment or placebo (i.e., saline, midazolam and electroconvulsive therapy).
(5). Type of outcomes: Studies that reported raw data or statistics related to SI as measured by any type of scale (e.g., SPS, ASIQ, CSSRS, MADRS-SI, BSI, QIDS-SI, etc.) were considered for inclusion.
Exclusion criteria
We excluded studies that met any of the following criteria:
(1). Type of studies: Abstracts of conference proceedings, study protocols, media news, commentaries, reviews, or case reports were excluded.
(2). Type of participants: Studies that did not report SI data were excluded.
(3). Type of interventions: Studies with unclearly reported interventions were excluded.
(4). Type of control: Studies with incomplete control group data were excluded.
(5). Type of outcomes: Studies with incomplete baseline data of participants were excluded.
Primary and secondary outcomes
The pre-defined primary outcome was the standardized mean difference (SMD) in SI scores measured by validated scales (e.g., CSSRS, MADRS-SI) between the ketamine and control groups at post-treatment.
Secondary outcomes included: (1) Prevalence of treatment-emergent adverse events (e.g., dissociation, nausea); (2) Moderator effects of age, administration treatment, frequency of medication, control, assessment tool, health status, administration route and study design.
Search strategy
In this systematic review and meta-analysis, two independent reviewers conducted a comprehensive search for studies evaluating the efficacy of ketamine in reducing SI. The search included various types of publications, including peer-reviewed publications and preprints, and was conducted across multiple databases (PubMed, Web of Science, medRxiv, EMBASE) using controlled vocabulary keywords such as ‘ketamine’, ‘racemic ketamine’, ‘esketamine’, ‘suicide ideation’ and ‘suicidal ideation’ (complete search syntax in Table S1).
Our inclusion criteria were restricted to studies published between January 2003 and October 2025 to capture the most contemporary evidence reflecting the paradigm shift in ketamine research following its Food and Drug Administration (FDA)-approval trajectory (U.S. Food and Drug Administration, 2019), evolving diagnostic criteria in DSM-IV-TR (Association, 2000), and standardized measurement tools in suicidology research (e.g., CSSRS 2003). When encountering multiple reports from the same cohort, we prioritized the largest/most complete dataset to prevent patient duplication. To ensure comprehensiveness, we conducted a thorough search for references cited in prior systematic reviews.
Study selection and data extraction
To ensure accuracy, we removed any duplicated studies, and then had two independent reviewers select the remaining studies based on the eligibility criteria. They examined the titles, abstracts, or full texts to make their selections. For each chosen study, we extracted sample size, participant characteristics, mean scores of SI, and the corresponding standard deviation (SD) to calculate the effect size. Safety outcomes were evaluated by the prevalence of adverse events. If data were unclear, we contacted the authors for clarification. If no response was received, the study was excluded. We combined data from multi-arm trials into a single comparison group. In cases of disagreement regarding study selection or data extraction, we resolved the issue through discussion or by consulting a third reviewer.
In order to investigate the correlation between ketamine treatment and SI, we collected additional information regarding trial characteristics and participants. The characteristics included the age, sex and health status of the participants, as well as the frequency, route, reaction time of administration, the proportion of adverse events, and whether the assessment was blinded to the intervention. The availability of SI measurement tools was also taken into account. We did not collect data regarding race and ethnicity as this was outside the study scope.
Assessment of bias and methodological quality
Two reviewers independently assessed the methodological quality of studies using the National Institutes of Health Quality Assessment Tools (National Health Lung and Blood Institute, 2021), selected for their ability to evaluate diverse study designs included in our analysis. The evaluation items in each tool included study participants, sample size justification, treatment allocation, measures of exposure and other biases. Each item was evaluated with possible answers being yes, no, or other. Disagreements were resolved either through discussion or with the assistance of a third reviewer. After the evaluation, we assigned each study a quality rating of ‘good’, ‘fair’, or ‘poor’. Regardless of the quality rating, all studies were treated equally in the primary analysis. In addition, we employed a funnel plot and used Egger and Begg’s test to assess the publication bias (or small-study effects).
Two independent reviewers assessed the quality of evidence in this review according to the Grading of Recommendations Assessment, Development and Evaluation approach (Atkins et al., Reference Atkins, Best, Briss, Eccles, Falck-Ytter, Flottorp, Guyatt, Harbour, Haugh, Henry, Hill, Jaeschke, Leng, Liberati, Magrini, Mason, Middleton, Mrukowicz, O’Connell, Oxman, Phillips, Schünemann, Edejer, Varonen, Vist, Williams and Zaza2004). The quality of evidence was rated as high, moderate, low, or very low based on several factors, such as study limitations, inconsistency, indirectness, imprecision and other relevant factors. In cases of disagreement, we resolved the issue through discussion or by consulting a third reviewer.
Data analysis
Using R and R Studio (Version 4.2.0), we conducted the meta-analysis by employing a random-effects model to calculate pooled estimates (SMD) and the corresponding 95% confidence interval (CI) to account for variations in participants and assessment instruments used for SI across studies. Adverse events were quantified as event prevalence with 95% CI. For each comparison, we estimated Cohen’s d using post-treatment or follow-up means, SDs and sample sizes, where a negative effect size suggested that ketamine therapy was more effective than the control condition or baseline. The safety of ketamine treatment was analysed using random-effects pairwise meta-analysis and was reported as prevalence and 95% CI. To assess between-study heterogeneity, we used the Cochran Q test. The percentage of variability that may be attributed to between-study heterogeneity rather than sampling error was calculated using the Higgins I 2 statistic, with I 2 values of 25%, 50% and 75%, respectively, denoting low, moderate and high levels of heterogeneity. Our meta-analysis accounted for variations across studies, using appropriate statistical tests to ensure accurate and reliable results (Higgins et al., Reference Higgins, Thompson, Deeks and Altman2003).
In order to evaluate potential publication bias, we employed two methods: a visual examination of the symmetry of the funnel plot and an Egger regression test. A P-value below 0.05 in the Egger regression test was indicative of significant publication bias. Moreover, we conducted sensitivity analyses by re-computing the effect size after eliminating possible outliers (Egger et al., Reference Egger, Davey Smith, Schneider and Minder1997). We classified studies as outliers if their 95% CI of effect size did not overlap with the 95% CI of the overall effect size (Viechtbauer and Cheung, Reference Viechtbauer and Cheung2010).
To assess the impact of potential moderators on the overall effect size and to explore any possible heterogeneity, we conducted meta-regressions. We selected three main moderators a priori: age of participants, evaluation time points and duration of medications. These moderators were chosen based on their possible associations with SI, ketamine treatment, or both and previous meta-analyses on ketamine treatment for SI in adults showed significant associations with similar moderators. To examine the impact of four primary moderators, we utilized multiple linear regression analyses with a mixed-effects model that employed maximum likelihood estimation. Furthermore, we conducted univariable analyses to explore whether participant and trial characteristics could moderate the overall effect. We considered a two-sided P-value of less than 0.05 to be significant. For detailed information on all moderators and their codes, please refer to eTable 1 in the Supplement.
Results
The initial database search yielded 1,669 unique records. After screening titles and abstracts, we assessed 104 full texts, and ultimately included 21 studies in the meta-analysis.
Study characteristics
The main characteristics of the included studies are summarized in Table 1. Of the 21 studies included, 13 were randomized double-blind controlled trials (RCTs), 8 were open-label studies, and included 927 participants (509 [54.9%] female; 418 [45.1%] male). The mean (SD) age of participants at baseline was 38.1 (13.2) years. Out of the 21 included studies, 16 studies enrolled participants with a psychiatric disorder, including depression, depressive disorder and treatment-resistant depression (TRD).
Table 1. Characteristics of included studies

ASIQ, Adult Suicidal Ideation Questionnaire; BSI, Beck Scale for Suicidal Ideation; CSSRS, Columbia-Suicide Severity Rating Scale; ECT, emission computed tomography; IM, intramuscular; IN, intranasal; i.v., intravenous; Ket, ketamine; MADRS-SI, Montgomery-Asberg Depression Rating Scale – Suicidal Ideation; MDD, major depressive disorder; MSSI, Modified Scale for Suicidal Ideation; PTSD, post-traumatic stress disorder; QIDS-SI, Quick Inventory of Depressive Symptomatology – Suicidal Ideation item; SI, suicide ideation; SPS, Suicide Probability Scale; TRD, treatment-resistant depression.
The frequency of the ketamine treatment ranged from one to four per week, with thrice weekly being the most commonly used frequency in repeated treatment (four studies [Phillips et al., Reference Phillips, Norris, Talbot, Hatchard, Ortiz, Birmingham, Owoeye, Batten and Blier2020; Sinyor et al., Reference Sinyor, Williams, Belo, Orser, Vincent, Mah, Zarate, Castel, Levitt and Schaffer2018; Zhou et al., Reference Zhou, Liu, Zheng, Wang, Zhan, Lan, Zhang, Zhang and Ning2020, Reference Zhou, Wang, Lan, Li, Chao, Wu, McIntyre and Ning2022]). Two studies evaluated intranasal administration of ketamine (Canuso et al., Reference Canuso, Singh, Fedgchin, Alphs, Lane, Lim, Pinter, Hough, Sanacora, Manji and Drevets2018; Peters et al., Reference Peters, Halpape, Cheveldae and Wanson2022), 1 study evaluated intramuscular injection (Kheirabadi et al., Reference Kheirabadi, Kheirabadi, Mirlohi, Tarrahi and Norbaksh2020), 1 study evaluated oral administration (Kheirabadi et al., Reference Kheirabadi, Kheirabadi, Mirlohi, Tarrahi and Norbaksh2020), and 18 studies evaluated the efficacy of intravenous infusion (Ahmed et al., Reference Ahmed, Elserogy, Elfadl, Ghada Abdelsalam and Ali2023; Fan et al., Reference Fan, Yang, Sun, Zhang, Li, Zheng and Liu2017; Feeney et al., Reference Feeney, Hock, Freeman, Flynn, Hoeppner, Iosifescu, Trivedi, Sanacora, Mathew, Debattista, Ionescu, Fava and Papakostas2021; Gaither et al., Reference Gaither, Ranney, Peachey, Burock, Rogers, Bucci and Beaudoin2022; Hochschild et al., Reference Hochschild, Keilp, Madden, Burke, Mann and Grunebaum2022; Hu et al., Reference Hu, Xiang, Fang, Zu, Sha, Shi, Ungvari, Correll, Chiu, Xue, Tian, Wu, Ma and Wang2016; Ionescu et al., Reference Ionescu, Bentley, Eikermann, Taylor, Akeju, Swee, Pavone, Petrie, Dording, Mischoulon, Alpert, Brown, Baer, Nock, Fava and Cusin2019; Kashani et al., Reference Kashani, Yousefian, Amini, Heidari, Younesian and Hatamabadi2014; Keilp et al., Reference Keilp, Madden, Marver, Frawley, Burke, Herzallah, Gluck, Mann and Grunebaum2021; Murrough et al., Reference Murrough, Soleimani, DeWilde, Collins, Lapidus, Iacoviello, Lener, Kautz, Kim, Stern, Price, Perez, Brallier, Rodriguez, Goodman, Iosifescu and Charney2015; Pathak et al., Reference Pathak, Ahuja, Dwivedi, Mishra, Kumar, Mishra and Singh2021; Phillips et al., Reference Phillips, Norris, Talbot, Hatchard, Ortiz, Birmingham, Owoeye, Batten and Blier2020; Price et al., Reference Price, Iosifescu, Murrough, Chang, Al Jurdi, Iqbal, Soleimani, Charney, Foulkes and Mathew2014; Shivanekar et al., Reference Shivanekar, Gopalan, Pizon, Spotts, Cruz, Lightfoot, Rohac, Baumeister, Griffo, Panny, Kucherer, Israel, Rengasamy and Price2022; Sinyor et al., Reference Sinyor, Williams, Belo, Orser, Vincent, Mah, Zarate, Castel, Levitt and Schaffer2018; Thakurta et al., Reference Thakurta, Das, Bhattacharya, Saha, Sen, Singh and Bisui2012; Zhou et al., Reference Zhou, Liu, Zheng, Wang, Zhan, Lan, Zhang, Zhang and Ning2020, Reference Zhou, Wang, Lan, Li, Chao, Wu, McIntyre and Ning2022). More information regarding the ketamine treatment of the included studies is available in eTable 2 in the Supplement.
Mean effect size, heterogeneity and bias
Figure 1 provides a visual representation of the distribution of the effect sizes of the included studies. It can be seen that none of the effect sizes is greater than 0, indicating larger reductions in SI with ketamine treatment compared to control conditions or baseline. The ketamine treatment had a mean effect size of −1.40 (SMD, 95% CI: −2.15 to −0.66, P < 0.001, low–quality evidence).

Figure 1. Forest plot of SMD in patients allocated to the ketamine group and the control group.
Only six studies assessed the effects of ketamine treatment on SI at follow-up (Feeney et al., Reference Feeney, Hock, Freeman, Flynn, Hoeppner, Iosifescu, Trivedi, Sanacora, Mathew, Debattista, Ionescu, Fava and Papakostas2021; Hu et al., Reference Hu, Xiang, Fang, Zu, Sha, Shi, Ungvari, Correll, Chiu, Xue, Tian, Wu, Ma and Wang2016; Shivanekar et al., Reference Shivanekar, Gopalan, Pizon, Spotts, Cruz, Lightfoot, Rohac, Baumeister, Griffo, Panny, Kucherer, Israel, Rengasamy and Price2022; Sinyor et al., Reference Sinyor, Williams, Belo, Orser, Vincent, Mah, Zarate, Castel, Levitt and Schaffer2018; Zhou et al., Reference Zhou, Liu, Zheng, Wang, Zhan, Lan, Zhang, Zhang and Ning2020, Reference Zhou, Wang, Lan, Li, Chao, Wu, McIntyre and Ning2022). The median follow-up period post-administration was 1 month (range, 2 weeks – 6 months). Efficacy in follow-up at Week 2 was −2.05 (SMD, 95% CI: −3.16 to −0.94, P < 0.001, low–quality evidence), indicating that ketamine was effective for alleviating SI at Week 2. No association of ketamine treatment with SI was detected in follow-up at Month 1 and Month 6 (Month 1: Cohen’s d = −1.417, 95% CI: −3.03 to 0.20, P = 0.086, very low–quality evidence; Month 6: Cohen’s d = −1.73, 95% CI: −5.60 to 2.14, P = 0.381, very low–quality evidence) (Figure S1). Overall, the differences were no longer statistically significant after 2 weeks (P > 0.05)
Only six studies assessed the effects of ketamine treatment on SI at follow-up (Feeney et al., Reference Feeney, Hock, Freeman, Flynn, Hoeppner, Iosifescu, Trivedi, Sanacora, Mathew, Debattista, Ionescu, Fava and Papakostas2021; Hu et al., Reference Hu, Xiang, Fang, Zu, Sha, Shi, Ungvari, Correll, Chiu, Xue, Tian, Wu, Ma and Wang2016; Shivanekar et al., Reference Shivanekar, Gopalan, Pizon, Spotts, Cruz, Lightfoot, Rohac, Baumeister, Griffo, Panny, Kucherer, Israel, Rengasamy and Price2022; Sinyor et al., Reference Sinyor, Williams, Belo, Orser, Vincent, Mah, Zarate, Castel, Levitt and Schaffer2018; Zhou et al., Reference Zhou, Liu, Zheng, Wang, Zhan, Lan, Zhang, Zhang and Ning2020, Reference Zhou, Wang, Lan, Li, Chao, Wu, McIntyre and Ning2022). The median follow-up period post-administration was 1 month (range, 2 weeks – 6 months). Efficacy in follow-up at Week 2 was −2.05 (SMD, 95% CI: −3.16 to −0.94, P < 0.001, low–quality evidence), indicating that ketamine was effective for alleviating SI at Week 2. No association of ketamine treatment with SI was detected in follow-up at Month 1 and Month 6 (Month 1: Cohen’s d = −1.417, 95% CI: −3.03 to 0.20, P = 0.086, very low–quality evidence; Month 6: Cohen’s d = −1.73, 95% CI: −5.60 to 2.14, P = 0.381, very low–quality evidence) (Figure S1). Overall, the differences were no longer statistically significant after 2 weeks (P > 0.05)
Only six studies assessed the effects of ketamine treatment on SI at follow-up (Feeney et al., Reference Feeney, Hock, Freeman, Flynn, Hoeppner, Iosifescu, Trivedi, Sanacora, Mathew, Debattista, Ionescu, Fava and Papakostas2021; Hu et al., Reference Hu, Xiang, Fang, Zu, Sha, Shi, Ungvari, Correll, Chiu, Xue, Tian, Wu, Ma and Wang2016; Shivanekar et al., Reference Shivanekar, Gopalan, Pizon, Spotts, Cruz, Lightfoot, Rohac, Baumeister, Griffo, Panny, Kucherer, Israel, Rengasamy and Price2022; Sinyor et al., Reference Sinyor, Williams, Belo, Orser, Vincent, Mah, Zarate, Castel, Levitt and Schaffer2018; Zhou et al., Reference Zhou, Liu, Zheng, Wang, Zhan, Lan, Zhang, Zhang and Ning2020, Reference Zhou, Wang, Lan, Li, Chao, Wu, McIntyre and Ning2022). The median follow-up period post-administration was 1 month (range, 2 weeks – 6 months). Efficacy in follow-up at Week 2 was −2.05 (SMD, 95% CI: −3.16 to −0.94, P < 0.001, low–quality evidence), indicating that ketamine was effective for alleviating SI at Week 2. No association of ketamine treatment with SI was detected in follow-up at Month 1 and Month 6 (Month 1: Cohen’s d = −1.417, 95% CI: −3.03 to 0.20, P = 0.086, very low–quality evidence; Month 6: Cohen’s d = −1.73, 95% CI: −5.60 to 2.14, P = 0.381, very low–quality evidence) (Figure S1). Overall, the differences were no longer statistically significant after 2 weeks (P > 0.05)
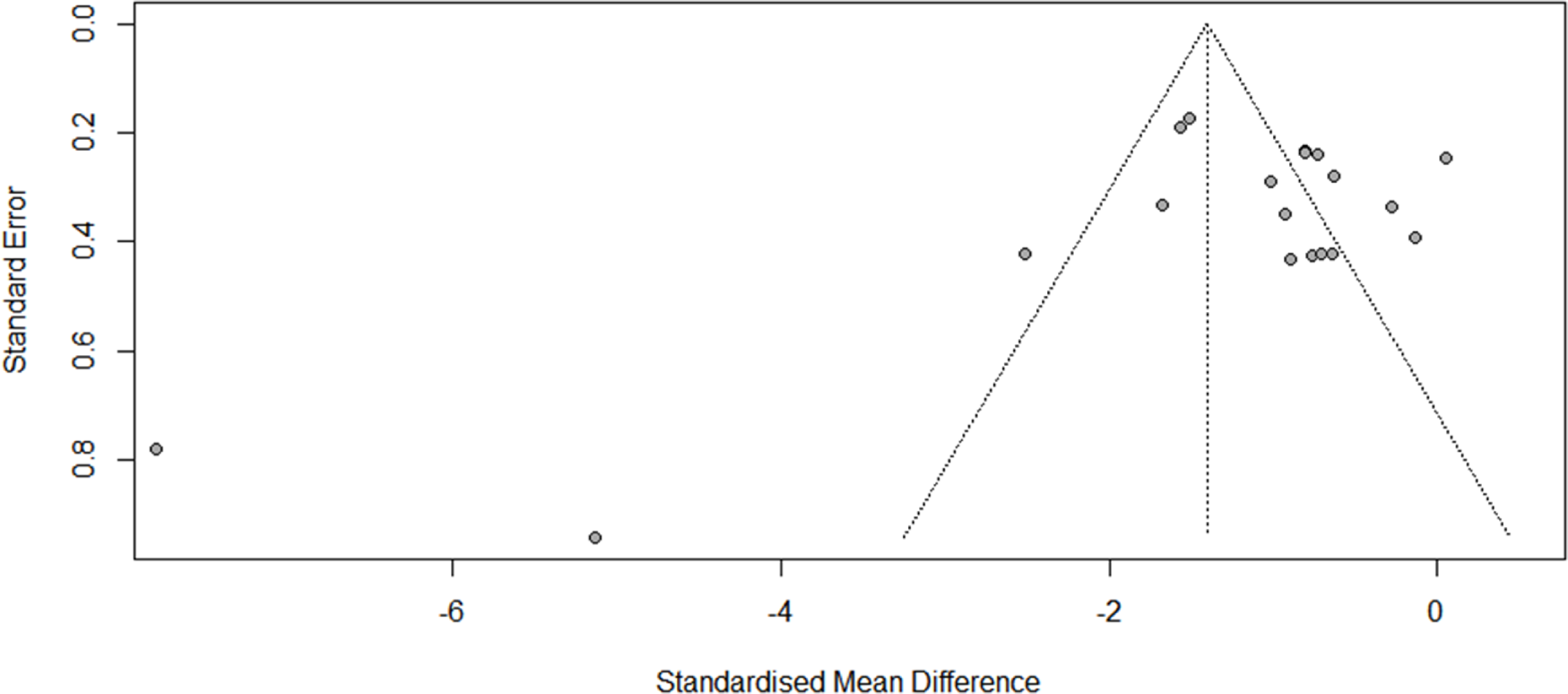
There was evidence of high heterogeneity at post-administration (I 2 = 89, P < 0.001) and at follow-up (Week 2: Q = 41.66; df = 3, I 2 = 92.8%, P < 0.001; Month 1: Q = 32.05; df = 3, I 2 = 90.6%, P < 0.001). An examination of publication bias was carried out by visual observation of the funnel plot in which the standard error of the SMD of each study was plotted against its SMD (Fig. 2). Egger’s regression test and Begg’s test suggested that there was no significant bias (Egger: −2.397, 95% CI: −6.072 to −1.277; t = −1.37, P = 0.187; Begg: −1.56, P = 0.119). Two outliers were detected (Pathak et al., Reference Pathak, Ahuja, Dwivedi, Mishra, Kumar, Mishra and Singh2021; Peters et al., Reference Peters, Halpape, Cheveldae and Wanson2022). Differences in effect sizes might be due to variations in the medication’s administration method or the usage of different measurement instruments. We conducted sensitivity analyses after excluding these studies, and the pooled effect size remained significant (Cohen’s d = −0.94; 95% CI: −1.25 to −0.64; P < 0.001; I 2 = 77.7%).

Figure 2. Funnel plots of the publication bias.
Risk of bias was evaluated for 13 double-blind RCTs, and 8 open-label studies; 14 received a good quality rating, and 7 received a fair quality grade (Table S3, Table S4). The absence of intention-to-treat in randomized individuals and the lack of sample-size justification in the trials were both frequent possible sources of bias in these investigations.
Moderator effects
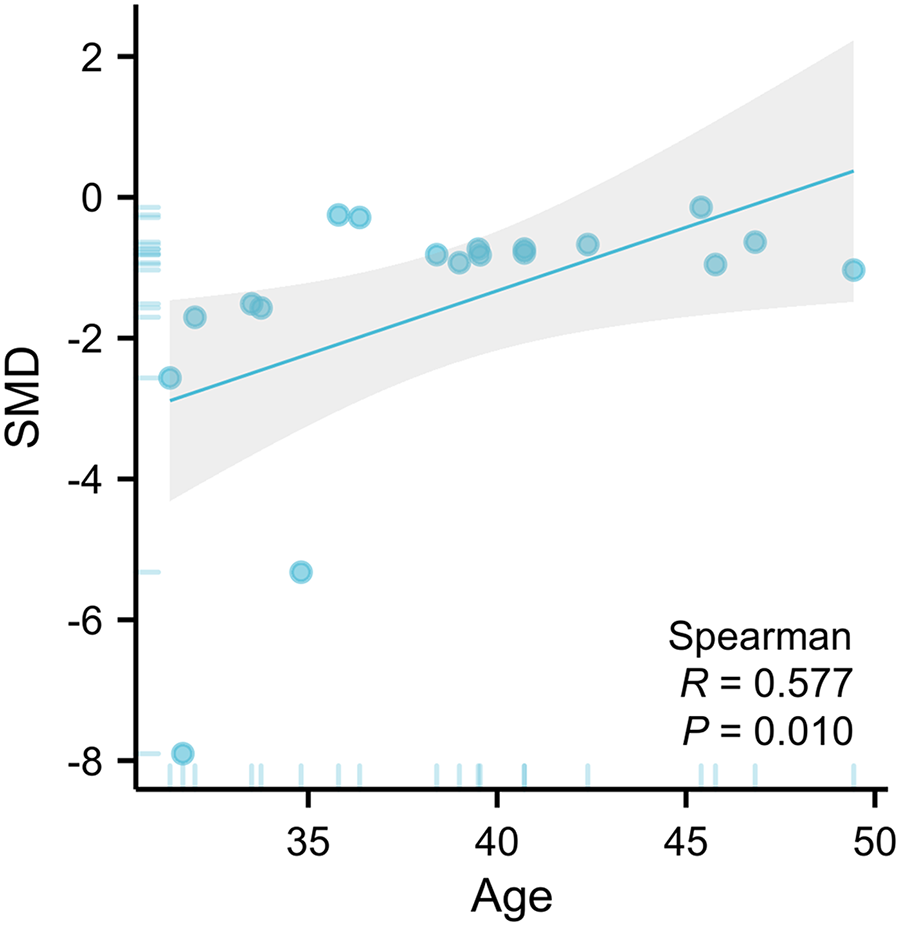
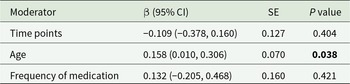
The primary moderator analysis confirmed that younger age significantly enhanced ketamine’s anti-suicidal efficacy (β = 0.158 per year, 95% CI: 0.01–0.31; P = 0.038, Fig. 3). The evaluation time points and the frequency of medication administered did not moderate the main treatment effect (Table 2; Figure S1).

Figure 3. An illustration of the association between age of participants and SMD.
Table 2. Moderator analyses

The bold values indicate statistical significance with p < 0.05.
Secondary analyses
Results of the secondary analyses are summarized in Table 3. In brief, ketamine treatment showed greater benefits in participants with a high risk of suicide. Health status of participants, study design and control medication also moderated the overall effect (P < 0.05). Open-label trials reported nearly double the effect size of double-blind RCTs (Cohen’s d = − 1.461 vs. − 0.697; P < 0.001), suggesting unblinding may amplify placebo effects or clinician-reported improvements. Similarly, studies with ‘no control’ arms overestimated efficacy (Cohen’s d = −1.554) compared to placebo-controlled trials (Cohen’s d = −0.671; P < 0.001), further supporting potential bias in uncontrolled designs.
Table 3. Secondary analyses

BSI, Beck Scale for Suicidal Ideation; CSSRS, Columbia-Suicide Severity Rating Scale; ECT, emission computed tomography; MADRS-SI, Montgomery-Asberg Depression Rating Scale – Suicidal Ideation; MDD, major depressive disorder; SI, suicide ideation; TRD, treatment-resistant depression. The bold values indicate statistical significance with p < 0.05.
Health status further stratified responses that patients with active SI exhibited the largest reductions (Cohen’s d = −2.107, 95% CI: −2.720 to −1.493), followed by those with TRD (Cohen’s d = −0.860) or major depressive disorder (MDD, Cohen’s d = −0.766).
Adverse events
The reported adverse events (such as nausea, dizziness, headache, anxiety, etc.) were used to gauge the ketamine’s safety. Dissociation (38.8%, 95% CI: 7.8%–69.8%, P = 0.014, very low–quality evidence), nausea (31.6%, 95% CI: 20.4%–42.8%, P < 0.001, very low–quality evidence), dizziness (24.7%, 95% CI: 8.7%–40.8%, P = 0.003, low–quality evidence), headache (22.0%, 95% CI: 5.1%–39.0%, P = 0.011, very low–quality evidence) and anxiety (15.8%, 95% CI: 8.2%–23.5%, P < 0.001, very low–quality evidence) were the most often reported side effects. The proportion of reported adverse events are summarized in Table S6.
Discussion
In this meta-analysis, we investigated the efficacy of ketamine treatment in reducing SI in high-risk populations. Our analysis of 21 studies showed a significant decrease in SI following ketamine treatment compared to placebo or other control conditions. However, the anti-suicide effects of ketamine were not sustained beyond a mean follow-up of 2 weeks. This finding contrasts with reports of longer-lasting antidepressant effects in other reviews (Li et al., Reference Li, Wang and Mei2022; Lima et al., Reference Lima, Visacri and Aguiar2022), though these conclusions derive from entirely distinct study cohorts (0/21 overlapping studies) focused on depression outcomes rather than SI. The certainty of evidence was downgraded to low due to substantial between-study heterogeneity (I 2 = 89%) and methodological limitations (e.g., 38% open-label designs). While sensitivity analyses excluding outliers confirmed robustness (SMD = −0.94; P < 0.001), generalizability to real-world settings remains constrained by stringent trial protocols.
Although the therapeutic effect of ketamine is significant, the precise mechanisms by which ketamine exerts its anti-suicidal effects are not yet fully understood. Ketamine rapidly inhibits indoleamine 2,3-dioxygenase (IDO-1), a key enzyme in the kynurenine pathway, within 4 hours of administration. This suppression reduces the production of neurotoxic quinolinic acid (QUIN)– a potent N-methyl-D-aspartate (NMDA)receptor agonist linked to glutamate excitotoxicity and neuronal apoptosis – while increasing KYNA, an NMDA antagonist with neuroprotective properties (Brown et al., Reference Brown, Christofides, Weissleder, Huang, Shannon Weickert, Lim and Newell2024; Savitz, Reference Savitz2020). Recent studies demonstrate that this acute rebalancing of QUIN/KYNA ratios correlates with rapid SI reduction in high-risk populations, independent of depressive symptom trajectories (Brown et al., Reference Brown, Christofides, Weissleder, Huang, Shannon Weickert, Lim and Newell2024). Kynurenine pathway modulation also explains ketamine’s transient efficacy: QUIN/KYNA ratios normalize within 2 weeks, mirroring the observed decline in anti-suicidal effects.
Our moderator analyses suggest that ketamine treatment has a more significant impact on reducing SI in younger populations than older ones. This finding is consistent with an RCT study that found that the effect of ketamine on reducing SI decreased with rising age in men (Derntl et al., Reference Derntl, Hornung, Sen, Colic, Li and Walter2019). One possible explanation for this age-related difference is that ketamine’s anti-suicide mechanism may involve modulating glutamatergic signalling, which may decline with age, leading to attenuated neuroplasticity pathways. This is supported by research showing that the integrity of signalling pathways in the hippocampus, such as those involving PPARα and F3/Contactin, is crucial for the action of antidepressants and can be compromised with age or stress (Chen et al., Reference Chen, Fan, Huang, Shi, Li, Wang, Jiang and Liu2022; Wang et al., Reference Wang, Gu, Liu, Liu, Tang, Ji, Guan, Zhao, Sun, Xu and Jiang2021). Therefore, age should be considered when designing treatment approaches for different age groups to optimize the effectiveness of ketamine therapy.
It is also worth noting that ketamine’s rapid onset of action may be particularly beneficial for individuals with active SI, as it can provide almost immediate relief from symptoms. This may be especially important for individuals at high risk of suicide, as they may not have the time to wait for traditional antidepressant medications to take effect.
Despite the promising results of this study, there are several limitations that should be acknowledged. The sample size of this meta-analysis was relatively small, which may limit the generalizability of the findings. In some comparisons, statistical heterogeneity was high. For this reason, a random-effects model was used to interpret the results of the comparisons with high heterogeneity, yet, future randomized studies with better designs will be required to further assess these outcomes. The majority of the studies included in this meta-analysis were conducted in a controlled setting with strict monitoring and supervision, which may not reflect real-world clinical practice. Finally, the long-term side effects of ketamine treatment for SI have not been fully evaluated, and further research is needed to address these issues. Our analysis revealed a notable gap in the assessment of long-term outcomes. Among the included studies, only one (Shivanekar et al., Reference Shivanekar, Gopalan, Pizon, Spotts, Cruz, Lightfoot, Rohac, Baumeister, Griffo, Panny, Kucherer, Israel, Rengasamy and Price2022) reported follow-up data extending to 6 months, and no studies systematically investigated potential long-term consequences such as the development of dependence, tolerance, or urinary tract toxicity with repeated administration. While acute adverse events (e.g., dissociation, nausea) were well-documented, the current evidence base is insufficient to characterize the long-term safety profile of ketamine for SI.
Conclusion
In summary, our meta-analysis offers support for the effectiveness of ketamine treatment in decreasing SI among high-risk individuals. Although further research is necessary to clarify the mechanisms underlying the effects of ketamine, the swift and powerful effects of this intervention on SI suggest that it holds considerable promise for high-risk patients. Nevertheless, clinicians must weigh the potential risks and long-term consequences of ketamine treatment carefully.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S2045796025100371.
Availability of data and materials
Extracted data are available on request to the corresponding author.
Author contributions
Wen Tang and Wei-Wei Jiang contributed equally as co-first authors. Hong-Lin Chen can also be contacted for correspondence, email honglinyjs@126.com.
Financial support
The authors received financial support from the Science and Technology Development Fund Project of Nanjing Medical University (NMUB20240244).
Competing interests
The authors declare no competing interests.
Ethical standards
Authors declare their research conforms to the journal's ethical standards.